
Dear colleagues!
Welcome to our belated spring news!
We are pleased to offer you a choice of articles on neuromuscular ultrasound that were published in the first quarter of 2022.
We are very excited about the things going on around NMUS. First and most important: two guidelines were published, which deal with the importance of NMUS in diagnostic work-up of our patients. The first is an expert consensus on electrodiagnostics (EDX) and NMUS in CTS, the second is an evidence-based guideline of NMUS for diagnosis of ulnar neuropathy at the elbow (UNE). So obviously you are on the right track if keeping up with us.
Further hot topics are the ultrasound appearance of devices used for nerve repair, the realization that after about 30 years of searching for the enlarged nerve, we are now reading more and more about disorders which lead to thinning of nerves and as always carpal tunnel syndrome.
We hope you’ll enjoy your read.

GUIDELINES
Pelosi et al. Expert consensus on the combined investigation of carpal tunnel syndrome with electrodiagnostic tests and neuromuscular ultrasound. Clin Neurophysiol 2022; 135: 107-16
Expert consensus on use of electrodiagnostic tests and neuromuscular ultrasound in diagnosing carpal tunnel syndrome was set up with the Dlephi method. There is agreement that combining EDX and NMUS is more informative than using each modality alone and that NMUS is especially helpful in patients with non-localizing or normal EDX, atypical EDX, failed CTS surgery, polyneuropathy and CTS suspected to be secondary to structural pathology.
EDX should always be performed for quantifying CTS and in patients over 70 years of age.
Shook et al. Evidence-based guideline: Neuromuscular ultrasound for the diagnosis of ulnar neuropathy at the elbow. Muscle Nerve 2022; 65(2): 147-53
Based on a literature review and class I evidence of four studies, it was concluded that NMUS measurements of the ulnar nerve at the elbow is accurate for diagnosing UNE. It is recommended that clinicians offer US measurement of the ulnar nerve at the elbow to diagnose and localize the site of compression (Level B).
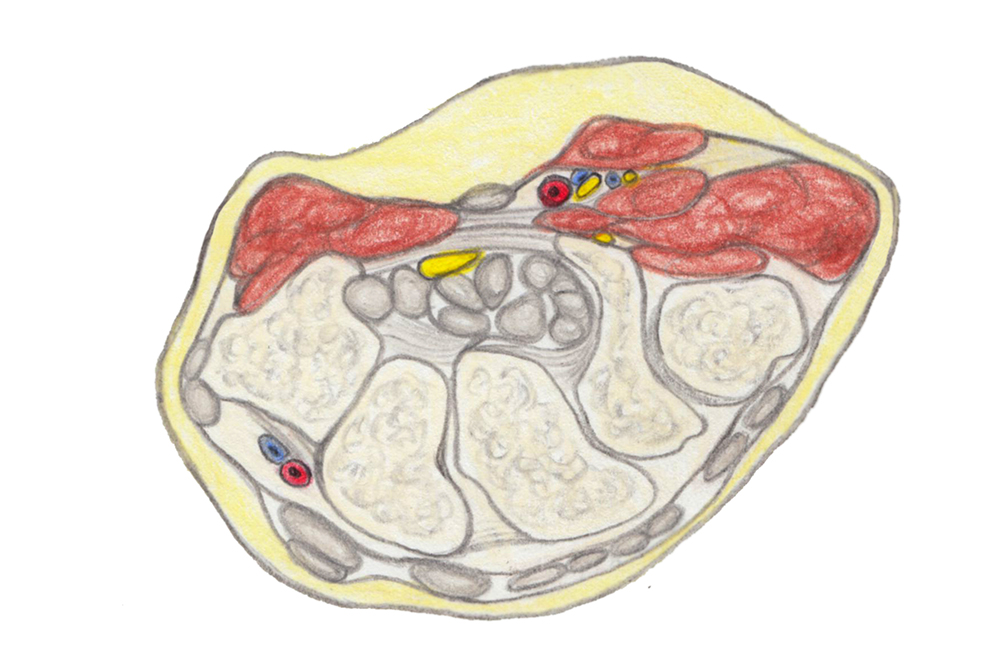
© Gerlinde Maria Gruber,2019
CARPAL TUNNEL SYNDROME & OTHER ENTRAPMENT NEUROPATHIES
Caritá et al. High-resolution ultrasound in the diagnosis of failed carpal tunnel decompression: a study of 35 cases. J Hand Surg Eur 2022; 47 (4):364-8.
35 patients with failed carpal tunnel release were examined with ultrasound to see if a cause could be identified. In 30 patients, persistent median nerve compression was found. In 20 patients this was caused by a residual transverse carpal ligament, in four by perineural fibrosis, in five by both and in one by tenosynovitis. Surgical findings confirmed ultrasound diagnosis in 29. The last one was a giant cell tumor and not a tenosynovitis.
NOTE: NMUS really assists you in failed carpal tunnel release.
Yao & Roll. An ultrasound study of the mobility of the median nerve during composite finger movement in the healthy young wrist. Muscle Nerve 2022; 65 (1): 82-88
The authors analyzed ultrasound videos of the median nerve during different positions of the hand and fingers in 197 healthy volunteers. In short axis, they noticed a movement within 1 mm vertically and 3 mm horizontally with no direction being predominant. 52% slid independently, 27% with the tendons and 21% not at all. There was a wide variety of nerve movement with no clear pattern of direction.
NOTE: This seems to question the contribution of reduced nerve sliding to e.g. symptoms in entrapment neuropathy.
Hsu et al. Median nerve injury in ultrasound-guided hydrodissection and corticosteroid injections for carpal tunnel syndrome. Ultraschall Med 2022; 43(2): 186-93
This study assessed frequency, symptoms and ultrasound indicators for median nerve injury (MNI) after US-guided hydrodissection with following corticosteroid injections. Of 126 patients with CTS 9 (7.1%) suffered a MNI, but all improved significantly at follow up. MNI was associated with a significant increase of CSA of over 2 mm2 at carpal tunnel inlet after hydrodissection.
Demino & Fowler. Prognostic value of ultrasound imaging of the median nerve in carpal tunnel syndrome. Hand 2022; 17(2):219-23
199 wrists of 172 patients who underwent carpal tunnel release were scanned before and regularly after surgery (2 weeks, 6 weeks, 6 months-one year). Measurements were correlated with the Boston Carpal Tunnel Questionnaire (BCTQ) (Note: a standardized questionnaire assessing symptom severity and functional impairment in patients with CTS). To sum up, higher preoperative CSA showed nearly no correlation with better outcomes after carpal tunnel release.

NERVE REPAIR
Bianci & Mauler. Ultrasound appearance of in vitro nerve allografts and conduits for peripheral nerve reconstruction. J Ultrasound Med 2022; 41(3): 763-71
Very interesting article dealing with the ultrasound appearance of devices currently used for bridging of nerve gaps after nerve trauma:
* human nerve allografts (decellularized and sterilized nerve segments, harvested from human cadavers)
* nerve conduits
– small tubules which provide a scaffold for the regenerating nerve
– can be made of or used as carriers for substances, which assist nerve regeneration and outgrowth
– based on their origin divided into:
synthetic nerve conduit (e.g. based on chitin of arthropods)
biological nerve conduit (vessels of the umbilical cord)
MOTOR NEURON DISEASE
Pelosi et al. Nerve ultrasound detects abnormally small nerves in patients with spinal and bulbar muscular atrophy. Muscle Nerve 2022; epub ahead of print Feb 14
For this prospective study, CSAs of median, ulnar, sural and tibial nerves were examined in seven patients with X-linked spinal and bulbar muscular atrophy (SBMA) and compared to those of 18 patients with neuropathy and 42 healthy controls. CSAs were significantly smaller in patients with SBMA compared to the two other groups. The most prominent difference was found at the upper limb, with CSAs in patients being only half of the size of controls and a third the size of neuropathy patients.
The authors conclude that the ultrasound changes are similar to those reported in other inherited sensory neuronopathies and that ultrasound allows a clear differentiation from axonal neuropathy.
Watanabe et al. Ultrasonographic evaluation reveals thinning of cervical roots and peripheral nerves in spinal and bulbar muscular atrophy. Neurol Sci 2022 Mar 2, online ahead of print
11 patients with genetically confirmed spinal and bulbar muscular atrophy (SBMA) and nine patients with ALS underwent ultrasound of C5 and C6 roots, the median nerve and the ulnar nerve. The roots as well as the median nerve at upper and forearm as well as the ulnar nerve at the upper arm were smaller in patients with SBMA than in patients with ALS. The authors hypothesized that this might be due to longer disease duration or additional sensory nerve involvement in patients with SBMA.
POLYNEUROPATHY
Niu et al. Nerve ultrasound performances in differentiating POEMS syndrome from CIDP. Neurotherapeutics 2022 Mar 7, online ahead of print
The authors examined 120 patients with CIDP and 34 patients with POEMS syndrome with nerve conduction studies (NCS; median and ulnar nerve) and ultrasound (median, ulnar, brachial plexus), looking for differences especially in ultrasound.
Half of the patients with CIDP were found to have a conduction block or probable conduction block in NCS, but none of the patients with POEMS. The main finding about ultrasound was that there was a bigger minimum-maximum variability for the median nerve in CIDP (2.8 +/- 2.8) patients than in the ones with POEMS (1.7 +/- 0.3).
A two-step protocol using conduction block and maximum/minimum CSA of the median nerve yielded a sensitivity of 93% and specificity of 79% for distinguishing the two diseases.
Niu et al. Nerve ultrasound may help predicting response to immune treatment in chronic inflammatory demyelinating polyradiculoneuropathy. Neurol Sci 2022, Jan 21, online ahead of print
This prospective cohort study included 89 therapy-naïve CIDP patients, who underwent a scan of median and ulnar nerves before treatment. In 45 patients also a follow up after treatment with steroids or IVIGs was available.
Treatment response differed significantly between patients with normal or moderate nerve enlargement vs. those with marked enlargement, with a better treatment response to steroids in the first group. Furthermore, a decrease in CSA at follow up was connected to a good IVIG/steroid response and no need for immune suppressant therapy.
Du et al. Vagus nerve ultrasound in transthyretin familial amyloid polyneuropathy: a pilot study. J Neuroimaging 2022; 32 (2): 285-91
As autonomic dysfunction is a common feature of transthyretin familial amyloid polyneuropathy (TTR-FAP), the study examined the CSAs the vagus nerve in 18 patients with TTR-FAP and compared them to 17 age-and gender-matched controls. The difference was significant on both sides with a mean of 3.5 mm2 for patients and 2.0 mm2 for controls on the right side and 2.5 mm2 vs. 1.0 mm2 on the left. CSA positively correlated with the course of disease, the composite autonomic score, left ventricular posterior wall thickness and interventricular septum thickness. The authors concluded that ultrasound of the vagus nerve may be an important clinical tool for assessing the severity of autonomic dysfunction in these patients.

SMALL NERVES
Abdelnaby et al. Sonographic reference values of v agus nerve: a systematic review and meta-analysis. J Clin Neurophysiol 2022; 39(1):59-71
This review analyses the sonographic reference values of the vagus nerve across all available studies (21 studies with 864 participants). Including the 95% confidence interval, the CSA ranged from 2.29 to 2.70 mm2 for the right and from 1.83 to 2.23 mm2 for the left vagus nerve. The mean CSA of 2.53 mm2 for the right and 2.03 mm2 for the left vagus is proposed as sonographic reference value in healthy adults.
Meng et al. Ultrasound of small nerves. Ultraschall Med 2022; 43(1): 12-33
This article reviews anatomy and scanning of a variety of small nerves, e.g. the great auricular nerve, the supraclavicular nerve, the medial antebrachial cutaneous nerve, etc.
Patel et al. Diaphragm and phrenic nerve ultrasound in COVID-19 patients and beyond. J Ultrasound Med 2022; 41 (2): 285-99
This review gives a good overview on scanning of the diaphragm and the phrenic nerve, also pointing out the problems with e.g. non-cooperative patients on the ICU.
Ok… now that was a lot to go through. We hope you liked it and can use some of the information for yourself. If you have any comments or we missed your most important paper, just leave us a comment!
Stay safe, see you soon.
Your Sonocampus Team.


Hi, thank you for your effort in collecting and reviewing the latest NMUS articles, I find it very useful. If you don’t mind I would like to ask you a question regarding the CIDP 2021 guidelines. The reference value for interscalene trunk is set there at 9mm2, however when it actually comes to calculating the CSA of the upper trunk (after C5+C6 join), I keep seeing CSA of the upper trunk >9mm2 in healthy people (usually around 13-15mm2). Middle trunk is okay but obviously it’s just a continuation of C7. What is your experience with this measurement? or maybe I’m doing something wrong and should be measuring interscalene C5 and C6 separately to conform with the guidelines? Thank you for your advice.
Dear Michal Blaz,
We are happy to hear that you enjoy our newsletter!
About the measurement of the trunks: you are completely right. This is a problem of use of currently non-standardized language in NMUS. If you take a look at the publications that led to the wording in the guidelines, you see that the authors actually refer to the roots in interscalene view as trunks, which is proximal to the formation of the upper trunk. This is surely a weakness we have to work on to avoid confusion! So thank you for this important question.
For my feeling also measurement of the upper trunk (C5 and 6 conjoined) is often helpful, but this has not been studied systematically.
Best regards
Doris
Thank you very much!